
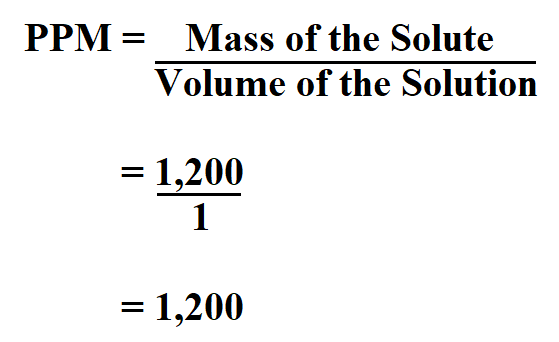
(% m/v), is commonly used for intravenous (IV) fluids ( Figure 9.2 "Mass/Volume Percent"). A hybrid concentration unit, mass/volume percent A concentration unit that relates the mass of the solute to the volume of the solution. (% v/v): the volume of a solute divided by the volume of a solution times 100: % v/v = volume of solute volume of solution × 100 %Īgain, the units of the solute and the solution must be the same. What is the mass/mass percent concentration of the solution?įor gases and liquids, volumes are relatively easy to measure, so the concentration of a liquid or a gas solution can be expressed as a volume/volume percent A concentration unit that relates the volume of the solute to the volume of the solution. If the solute is a gas, it can bubble out of solution uncontrollably, like what happens when you shake a soda can and then immediately open it.Ī dextrose (also called D-glucose, C 6H 12O 6) solution with a mass of 2.00 × 10 2 g has 15.8 g of dextrose dissolved in it. If the solute is solid, excess solute can easily recrystallize. Under special circumstances, more solute can be dissolved even after the normal solubility limit is reached such solutions are called supersaturated and are not stable. If a solution contains less solute than the solubility limit, it is unsaturated A solution whose solute is less than its solubility limit. If a solution contains so much solute that its solubility limit is reached, the solution is said to be saturated A solution whose solute is at its solubility limit., and its concentration is known from information contained in Table 9.2 "Solubilities of Various Solutes in Water at 25☌ (Except as Noted)". Table 9.2 Solubilities of Various Solutes in Water at 25☌ (Except as Noted) Substance


 0 kommentar(er)
0 kommentar(er)
